A History of the cathode rays and some more undeserved Nobel prizes
Science and technology exposed
under construction
For more info on this subject see the ‘Electron Deception’ page.

“Any theory will account for some facts; but only the true explanation will satisfy all the conditions of the problem …”. William Crookes.
This blog and part 2 follows the now well trodden path taken when investigating the history of radio, the electron, radio astronomy etc., to find a true-as-is-possible history of television invention and development. To ask about the actual pioneers, as opposed to mythical revisionist contributions made by academic science; to dispel myths attached to well known names and fabricated scientific heroes. To highlight those deserving of the accolades.
By far the most integral part of a TV development was the cathode ray tube and in the following Wiki article, we see that J.J. Thomson, an English physicist is mentioned first and the authentic developer, chemist William Crookes, who did most of the work is mentioned last. This is scientific revisionism and its habit of claiming all research and invention for its beloved sons… Crookes was also an occultist, something guaranteed to exclude him from the roster of the great and good in science.
Wiki: “The experimentation of cathode rays is largely accredited to J.J. Thomson, an English physicist who, in his three famous experiments, was able to deflect cathode rays, a fundamental function of the modern CRT. The earliest version of the CRT was invented by the German physicist Ferdinand Braun in 1897 (untrue) and is also known as the Braun tube. It was a cold-cathode diode, a modification of the Crookes tube with a phosphor-coated screen.” 1
It’s all lies so let us see what really happened…
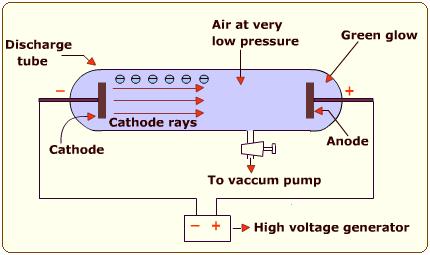
The Technology
Vacuum Discharge Tubes
arcsandsparks.com: A vacuum discharge tube is a glass vessel into which metal electrodes have been sealed and from which the air has been removed by a vacuum system. The earliest forms of such tubes appeared in the late 17th century but, although experimenters like Jean Picard, Francis Hauksbee, William Morgan, and even Michael Faraday experimented with vacuum discharge tubes, it was not until the 1850s that sufficient technology existed to produce sophisticated versions of such tubes. This technology included efficient vacuum pumps, advanced glassblowing techniques, and the Ruhmkorff induction coil. http://www.arcsandsparks.com/aboutvacuumtubes.html

Around 1650 Otto von Guericke invented the vacuum pump and in 1671 he published his treatise “Experimenta nova Magdeburgia de vacuo spatio” or “New Magdeburg Experiments About the Vacuum”. As a result researchers were able to experiment with rarefied air and electricity. Circa 1705 it was noted by experimenters that electrostatic generator sparks travel a longer distance in rarefied air than in standard air. In 1838 when more was understood about the electrical process the self educated Michael Faraday passed a high current through rarefied air in a glass tube and noticed a strange light, an arc with its beginning at the cathode (negative electrode) and its end almost at the anode (positive electrode). The only place with no luminescence was just in front of the cathode, which came to be called the “cathode dark space” or “Faraday dark space” or “Crookes dark space”. It became known that when a voltage is applied to a tube containing rarefied air, a light is produced. Although Faraday has been elevated to the pinnacle of scientific greatness, contemporary writers and attendees at his lectures tell us that he freely admitted to gleaning his ideas from others. He may well have been a brilliant experimenter, but the waters have been so muddied by modern scientific revisionists of all political colours that the truth is hard to discern.

Wiki tells that: Heinrich Daniel Ruhmkorff (Rühmkorff) (January 15, 1803 in Hanover – December 19, 1877 in Paris) was a German instrument maker who commercialised the induction coil (often referred to as the Ruhmkorff coil.) The induction coil was used to create the high voltage needed to energise the vacuum tube.
We need next to look at the voluminous and invaluable contribution made by William Crookes and at that time his more important X-ray and nuclear research during the
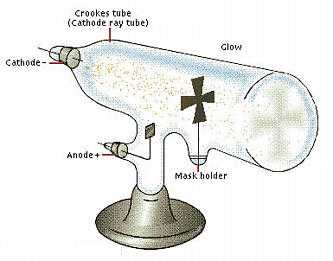
development of Crooks type tubes that eventually became the cathode ray tubes used in TV. The cathode ray tube was also the forerunner of what was to become the particle accelerator, being used in the early days in just the same way as the Large Hadron Collider technology of today. In fact colliders are a development of cathode ray tubes. Although we will see that academic physicists were involved in cathode ray experimentation, they were only able build their reputations on the firm foundation provided by the almost unheard of today, chemist and psychical researcher William Crookes. Historically, this most important component of electronic TV was used in all TV sets and monitors until it was replaced by the flat screen display. The vacuum tube or valve may or may not have evolved from the Crookes tube, I have found no connection even though they work on the same principle. The vacuum tube was used in radio and later in TV for amplification, switching and rectification, to be eventually replaced by the transistor, yet another important technology like so many within these pages, originating from the work of non-academic inventors prior to the 1930’s, a time when everything in science changed.

Robert H. Goddard of US rocket fame produced a vacuum tube much like a cathode ray tube but Goddard, for whatever reason, was a joke as far as the US press of the day was concerned and his idea was ignored.
geni.com: Dr. Robert H. Goddard vacuum tube First patents:
In the decades around 1910, radio was a new technology, a fertile field for innovation. In 1911, while working at Clark University, Goddard investigated the effects of radio waves on insulators.[36] In order to generate radio-frequency power, he invented a vacuum tube that operated like a cathode-ray tube. US patent 1,159,209 was issued on November 2, 1915. This was the first use of a vacuum tube to amplify a signal, preceding even Lee de Forest’s claim. https://www.geni.com/people/Robert-H-Goddard/6000000010636990983
Lee de Forest produced what became known as the triode vacuum tube, although he admitted that he did not know how it worked. These things are something of a mystery as far as history is concerned.

William Crookes
who was self taught, is now called a physicist, but he was educated to be a chemist and he became an amateur physicist, something that does not exist today primarily because such persons are not taken seriously by academia. He was also a spiritualist and a member of the Society for Psychical Research, an interest that would certainly cause concern for the materialist humanist and sceptical denizens of the scientific community of our day. If Crookes were alive in our time he would be referred to as a pseudo-scientist or at best a ‘tinkerer’, a derogatory expression when used by a physicist that denotes a departure from theory. But let’s look at his tinkering? 2
Crookes and Radioactivity
Wiki: “In his (Crookes’) investigations of the conduction of electricity in low pressure gases, he discovered that as the pressure was lowered, the negative electrode (cathode) appeared to emit rays (the so-called cathode rays, now known to be a stream of free electrons, and used in cathode ray display devices). Crookes investigated the properties of cathode rays, showing that they travel in straight lines, cause phosphorescence in objects upon which they impinge, and by their impact produce great heat. As these examples indicate, he was a pioneer in the construction and use of vacuum tubes for the study of physical phenomena. He was, as a consequence, one of the first scientists to investigate what are now called plasmas. He also devised one of the first instruments for the study of nuclear radioactivity, the spinthariscope.” 3 4

Wiki: ”In 1903, Crookes turned his attention to the newly discovered phenomenon of radioactivity, achieving the separation from uranium of its active transformation product, uranium-X (later established as protactinium). He observed the gradual decay of the separated transformation product, and the simultaneous reproduction of a fresh supply in the original uranium. At about the same time as this important discovery, he observed that when “p-particles”, ejected from radio-active substances, impinge upon zinc sulfide, each impact is accompanied by a minute scintillation, an observation which forms the basis of one of the most useful methods in the technique of radioactivity.” 5
Wiki: “In 1861, Crookes discovered a previously unknown element with a bright green emission line in its spectrum and named the element thallium. Crookes also identified the first known sample of helium in 1895. He was also the inventor of the Crookes radiometer.” 6 7 8
Encyclopædia Britannica, Cathode Ray Tube:

“…he (Crookes) devoted himself from 1856 entirely to scientific work of various kinds at his private laboratory in London. His researches on electrical discharges through a rarefied gas led him to observe the dark space around the cathode, now called the Crookes dark space. He demonstrated that cathode rays travel in straight lines and produce phosphorescence and heat when they strike certain materials.” 9
discoveriesinmedicine.com tells us: It was in 1838 that Michael Faraday had first described the characteristics of the gas discharge tube, a glow witnessed in a partially evacuated tube with an applied a high voltage. But,”Probably the most important research using cathode-ray tubes was performed in 1875 by the English physicist William Crookes. In order to confirm the experiments of Plucker and Hittorf, Crookes designed his own vacuum tube from which the air could be almost completely removed. So great an improvement over Geissler’s tubes were these that the “Crookes tube” quickly became the standard vacuum tube for use in scientific experiments. Crookes continued Plucker’s experiments with magnetic fields, confirming the glow was easily deflected.(See J.J.Thomson below who is said to be the first to achieve this) He also installed tiny vanes within his tubes. As the current was applied the vanes would turn slightly (it was as if they were blown by a gust of wind).” 10 11
Heinrich Geissler

discoveriesinmedicine.com again: “The German team of Heinrich Geissler (1815-1879) and Julius Plucker (1801-1868) pioneered the study of cathode-ray tubes. Geissler was a skilled glassworker, employed by the University of Bonn (Germany) as a maker of scientific instruments. 12
Wiki informs us that “Geissler was awarded an honorary doctorate in 1868.” He did not have a qualification in physics when he did his important work and was not a physicist as stated in other Wiki articles. 13 Crooks tubes were being produced on an almost industrial scale at the time. And so the stage was set for experiments with cathode ray tubes.
The Paradox
This is the point where the scientists took-over and it’s interesting to note that all of the electronics required for J J Thomson’s ‘electron discovery’ were in place. We see that there were resistors, capacitors, inductors and transformers, cathode ray tubes and vacuum tubes, in fact all the components of a modern electronic circuit are present before the electron discovery. But there is little if any mention of any of them ever suggesting the picture tube that would become television. It’s all about the scramble for a new branch of science, particle physics, increased funding and a seemingly unnecessary electron.

Johann Wilhelm Hittorf
discoveriesinmedicine.com says: “The next scientist to conduct important research using vacuum tubes was Johann Hittorf (1824-1914) in 1869″… 14 Hittorf was a scientist but did little to advance the cathode ray tube, his most noted work being in the area of ion movement using the Crookes tube in his experiments. “He noticed that when there was any object placed between the cathode and the illuminating side of the tube, then the shadow of that object appeared.” Something already done by William Crookes. 15
Eugen Goldstein
“German scientist Eugen Goldstein (1850-1930) first dubbed Crookes’s rays “cathode rays” in 1876.” …and little else of benefit to TV or cathode ray tube development. 16

Phillip Lenard
Wiki and some questionable English: “As a result of his Crookes tube investigations, he showed that the rays produced by irradiating metals in a vacuum with ultraviolet light were similar in many respects to cathode rays. His most important observations were that the energy of the rays was independent of the light intensity, but was greater for shorter wavelengths of light. These latter observations were explained by Albert Einstein, whose Serbian wife, Mileva Maric, worked in Lenard’s lab for a time, as a quantum effect.” 17
I don’t think Einstein’s wife “worked as a quantum effect”, it was the ultraviolet light.
discoveriesinmedicine.com: “In 1892, Phillip Lenard (Nobel Prize winner for work oncathode rays.) followed up on Heinrich Hertz’s discovery that under certain conditions cathode rays could penetrate metal (X-rays?). Lenard succeeded in passing cathode rays through a window of thin metal set into the side of a Crookes tube. The rays exited the tube through the window into the air. Lenard proved that cathode rays were not a phenomenon exclusive to a vacuum. While performing a similar experiment in 1895, the German physicist Wilhelm Roentgen (1845-1923) accidentally discovered an even more penetrating form of radiation, which he called X-ray radiation.”. 18 19 And so, Roentgen, so beloved by educators and the scientific community, had discovered something already discovered by Lenard, Tesla and Hertz as we will see below, but because they failed to actually call them x-rays, they were disqualified from the discovery? Well this is the tale we are told!
And then we find that…
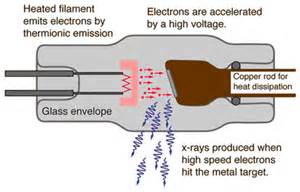
Wiki: “In April 1887, five years before Lenard, Nikola Tesla began to investigate X-rays using high voltages and tubes of his own design, as well as Crookes tubes. From his technical publications, it is indicated that he invented and developed a special single-electrode X-ray tube, which differed from other X-ray tubes in having no target electrode. The principle behind Tesla’s device is called the Bremsstrahlung process, in which a high-energy secondary X-ray emission is produced when charged particles (such as electrons) pass through matter. By 1892, Tesla performed several such experiments, but he did not categorized the emissions as what were later called X-rays. (Disqualified for failing to use the correct phrase ‘X-ray’?)
Tesla generalized the phenomenon as radiant energy of “invisible” kinds. He stated the facts of his methods concerning various experiments in his 1897 X-ray lecture before the New York Academy of Sciences. Also in this lecture, Tesla stated the method of construction and safe operation of X-ray equipment. His X-ray experimentation by vacuum high field emissions also led him to alert the scientific community to the biological hazards associated with X-ray exposure.” 20 Tesla was vilified and called a madman by academic science.

Wiki: ”Wilhelm Conrad Roentgen (1845 1923) was a German physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range today known as X-rays or Roentgen rays, an achievement that earned him the first Nobel Prize in Physics in 1901.” Yet again the prize goes to someone other than the discoverer. 21 The reader will recall that Tesla was producing X-rays in 1887.
Tesla researched X-rays for some time before Roentgen, his lab’ and X-ray research being destroyed in the year that Rontgen made his first X-ray discovery: Wiki: “Tesla continued research in the field, he performed several experiments prior to Roentgen’s discovery (including photographing the bones of his hand. Later, he sent these images to Roentgen) but did not make his findings widely known; much of his research was lost in the 5th Avenue laboratory fire of March 1895.” 22 Roentgen was well aware of Tesla’s priority, in fact, Tesla was lecturing on existing X-ray equipment, his experiments already completed and even to the extent of health and safety issues only two years after Roentgen’s so called discovery in 1895.
And unsurprisingly we find at Wikiradiography.com: “Historically, the first X-ray tube was invented by Sir William Crookes. It was used to make a visible fluorescence on minerals.” 23

Karl Ferdinand Braun
crtsite.com: “…Braun a German physicist, interested in the just discovered Cathode rays worked and experimented with Crookes tubes. When he was working at the Physics Institute of the Strasbourg University (from 1895-1918) he developed the first cold Cathode Ray tube with magnetic beam deflection (the same effect discovered by Plucker and Hittorf in 1869 and the cold cathode dates back to Faraday.) and a mica screen covered with phosphor to produce a visible spot. (The phosphorescence already discovered by Crookes) The tube, built for him by Franz Muller successor to Geissler was called after its inventor, the Braun tube. Braun used this tube as an indicator tube for studying the effects of Cathode rays in order to visualize alternating currents (AC generation discovered by Tesla) and described this in 1897, this was in fact the first oscilloscope.” 24 XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
There is some interesting info on Crookes at crtsite.com and here below we see that he was experimenting with fluorescent and phosphorescent minerals in 1881 well before Braun:
Here is a list of some common used minerals in Crookes tubes.
Color mineral
red calcite
yellow apatite
bright green willemite
blue scheelite
brown dolemite
violet magnesite
http://www.crtsite.com/page7.html
inventors.about.com: “In 1897 Braun introduced a CRT with a fluorescent screen, known as the cathode ray oscilloscope.” 25
4.nau.edu “Crookes also discovered that some minerals would glow if placed in the tube. This is the phenomenon of cathodoluminescence (production of visible light by electron bombardment”…) 26
crtsite.com: “The Braun tube, (See the illustration at the link) this small early 1900 tube is in fact a cold Cathode Crookes tube with an internal mica screen covered with phosphorescent paint.” 27 It’s hard to point a finger at the contribution made by Braun. He used a Crookes tube and added a screen of material that had been researched by Crookes, using beam deflection by Plucker and Hittorf for ‘his’ oscilloscope? He gave the world the little screen with the green glow and the wiggly line, so beloved in early science fiction movies that added a missing mystique to science and the work of TV repair men.
Although none of these scientists made any direct contribution to TV the Braun oscilloscope set the pattern for a phosphorescent screen at the end of the tube that would eventually become the TV picture.
Heinrich Hertz
dartmouth.edu: “Heinrich Hertz, a leading German experimentalist, tried to deflect cathode rays with an electric field, but was not able to do so. Since he knew that charged particles are deflected by electric fields, Hertz concluded that cathode rays were not charged particles, but waves that could be deflected with magnetic fields.” 28 (There is more on this at Electron Deception 1)

J.J. Thomson
Wiki: “The experimentation of cathode rays is largely accredited to J.J. Thomson, an English physicist who, in his three famous experiments, was able to deflect cathode rays, a fundamental function of the modern CRT.” 29
Wiki continues: “In May–June 1897 Thomson investigated whether or not the rays could be deflected by an electric field. Previous experimenters (Hertz) had failed to observe this, but Thomson believed their experiments were flawed because their tubes contained too much gas.” 30
The New World Encyclopaedia says:
“In his second experiment, he investigated whether or not the rays could be deflected by an electric field (something that is characteristic of charged particles). Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because they contained trace amounts of gas. Thomson constructed a cathode ray tube with a practically perfect vacuum, and coated one end with phosphorescent paint. Thomson found that the rays did indeed bend under the influence of an electric field.” 31
The Cambridge Cavendish Museum tells us:
“Eventually Thomson realised that the absence of electric deflection was due to the residual gas in the tube being ionised by the rays. The ions were attracted to the electric plates and cancelled out the applied field. ‘The thing to do was to get a much higher vacuum. This was more easily said than done… However after running the discharge through the tube day after day without introducing fresh gas, the gas on the walls and electrodes got driven off and it was possible to get a much better vacuum. The deflection of the cathode rays by electric forces became quite marked…” 32 Is it possible to improve a vacuum by “running the discharge day after day”, where does the gas go?
Thomson’s Cathode Ray Tube and TV
Both the Thomson electrostatic deflection and earlier electromagnetic deflection tubes were used for future applications. The Thomson electrostatic CRT being an alternative used mainly for oscilloscopes rather than an improvement. web.lemoyne.edu: “In all modern CRT monitors and televisions, the beams are bent by magnetic deflection, a varying magnetic field generated by coils and driven by electronic circuits around the neck of the tube, although electrostatic deflection is commonly used in oscilloscopes, a type of diagnostic instrument.” 33
The electrostatic plates of Thomson were for the electrical measurements involved in his sub-atomic studies and not CRT improvements. His electrostatic CRT system was not in general use for TV tubes although it was used occasionally.
From the notes of Thompson’s Nobel lecture:
“William Crookes was a productive researcher and highly original and speculative thinker in many areas of physics and chemistry. (See chapter 14, note 29.) His work on electrical discharges in vacuum tubes in the late 1870s laid some foundational work on which Thomson built; indeed, his “Crookes tubes” were widely used in cathode ray research…” 34
What the above is really saying, is that Crookes had already done the research. Thomson was not interested in improving cathode ray tubes, so much as making a name for himself in the field of subatomic physics – for which he received the Nobel prize.
 Discovery of the Electron
Discovery of the Electron
Wiki: “He was awarded a Nobel Prize in 1906, “in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases.” 35
Again, he was following-up on the plasma work started by Crookes. When stealing the thunder of previous inventors, the sons of science retreat into an obscure theoretical quasi-land where anything goes and are thereby able to receive the prestigious honours that should have gone to those more deserving.
Theories at the time of discovery
egglescliffe.org.uk: “One theory was that the rays were waves travelling in an invisible fluid called the “ether.” At that time, many physicists thought that this ether was needed to carry light waves through apparently empty space. Another possibility was that cathode rays were some kind of material particle. Yet many physicists, including J.J. Thomson, thought that all material particles themselves might be some kind of structures built out of ether, so these views were not so far apart.” 38 The ‘discoverer’ of the electron was an ether theory man who would be laughed to scorn by the science of today. But the discovery and theory of the electron certainly arose from technology based on, and emerging out-of, the debunked ether theory. The author wants an explanation of this paradox from an academic?
Richard Laming
Wiki also says that: Between 1838 and 1851 “he (British surgeon Richard Laming c.1798-1879) published a series of papers speculating about the electrical makeup of atoms. He hypothesized that there existed sub-atomic particles of unit charge; perhaps one of the first persons ever to do so.” 39
1890. Arthur Schuster
Wiki: “The German-born British physicist Arthur Schuster expanded upon Crookes’ experiments by placing metal plates parallel to the cathode rays and applying an electric potential between the plates. The field deflected the rays toward the positively charged plate, providing further evidence that the rays carried negative charge. By measuring the amount of deflection for a given level of current, in 1890 (Arthur) Schuster was able to estimate the charge-to-mass ratio of the ray components. However, this produced a value that was more than a thousand times greater than what was expected, so little credence was given to his calculations at the time.” 42
But precedence was given to Thomson for exactly the same thing.
1891 George Johnstone Stoney
Wiki: (Astronomer), “Stoney’s most important scientific work was the conception and calculation of the magnitude of the “atom of electricity”. In 1891, he proposed the term ‘electron’ to describe the fundamental unit of electrical charge, and his contributions to research in this area laid the foundations for the eventual discovery of the particle by J.J. Thomson in 1897.” 40
experiment-resources.com: 1897. “Thomson found out that the charge to mass ratio was so large that the particles either carried a huge charge, or were a thousand times smaller than a hydrogen ion. He decided upon the latter and came up with the idea that the cathode rays were made of particles that emanated from within the atoms themselves, a very bold and innovative idea.”41
Bold in as much as it was someone else’s idea, and innovative in that it won him a Nobel prize. Unlike Stoney and Schuster below, the actual originators of the idea, he decided to stick with the results of the experiments of others and bluff-it-out.
chemteam.info: “1890 Arthur Schuster calculates the ratio of charge to mass of the particles making up cathode rays (today known as electrons) by measuring the magnetic deflection of cathode rays and Joseph John (J.J.) Thomson first becomes interested in the discharge of electricity through a gas at low pressure, that is to say, cathode rays.” 43
Thomson and Arthur Schuster attended the same college, did the same research and knew all about each-others work. The American Association of Physics Teachers think that Thomson was awarded the Nobel because he was a better experimental physicist than Schuster. 44 It seems that Nobel prize winners require an intangible something, “The Right Stuff”, that has little to do with contribution, originality or ideas. Just the staying power required whilst being transformed into a scientific celebrity.
The rays had already been deflected by a magnet and as magnetism and electricity are part of the same phenomenon it’s a fair bet to say that an electric field should also deflect the cathode rays or there is something seriously amiss with electronic theory.
chemteam.info: “At this time there was great rivalry between German and British researchers. As concerning the nature of the cathode ray, the Germans tended to the explanation that cathode rays were a wave (like light), whereas the British tended to believe that the cathode ray was a particle. As events unfold over the next few decades, both will be proven correct. In fact, J.J. Thomson will be awarded the Nobel Prize in Physics in 1906 for proving the electron is a particle and his son, George Paget Thomson, will be awarded the Nobel Prize in Physics in 1937 for showing that the electron is a wave.” 45 We are thus made aware of the flimsiness of scientific rationale and how much it depends upon reputations. But sometimes reputations are constructed on even less evidence:
dartmouth.edu: “Heinrich Hertz, a leading German experimentalist, tried to deflect cathode rays with an electric field, but was not able to do so. Since he knew that charged particles are deflected by electric fields, Hertz concluded that cathode rays were not charged particles, but waves that could be deflected with magnetic fields.” 46
lemoyne.edu: “The case of the electron raises several interesting points about the discovery process. Clearly, the characterization of cathode rays was a process begun long before Thomson’s work, and several scientists made important contributions. In what sense, then, can Thomson be said to have discovered the electron? After all, he did not invent the vacuum tube or discover cathode rays. Discovery is often a cumulative process. The credited discoverer makes crucial contributions to be sure, but often after fundamental observations have been made and tools invented by others. Thomson was not the only physicist to measure the charge-to-mass ratio of cathode rays in 1897, nor the first to announce his results. (See Pais 1986.) But Thomson did carry out this measurement and (later) the measurement of the particles’s charge, and he recognized its importance as a constituent of ordinary matter.” 47
As we see from the above, the early cathode ray tube, that went on to become a particle accelerator for physicists and a TV tube for technologists was used to enhance the reputations of academic scientists in the field of theoretical particle physics and by default it became their property.
None of the physicsts saw its potential as a picture tube as this would have been technology, something anathema to academic science, that prefers obscure theory. The wholesale reassignment of ideas, and failure to give credit for the ideas of non-academic researchers is the rule and not the exception.
There is a huge difference between researchers/discoverers like Crookes and Tesla and academic scientists like J J Thomson, in that the former gave us a technology to prove every theory, whereas the latter did not think this was necessary.
Has the knowledge that an electron behaves like both a particle and a wave advanced technology? I think it would be difficult to say that it has, but science fosters the myth that it will someday be of use. The weapons of obscurity and inscrutability are what keep academics in a job.
When you switch on an electric light, the light does not use-up electrons like putting coal on a fire. When an electric motor is switched on, it does not use-up electrons like petrol in the fuel tank of a car. No electron is changed or used-up, they don’t alter at all. If the electron exists, it is as a carrier of energy, an energy that remains completely unexplained and unidentified by science.
There is a web site that graphically demonstrates an electric current fed into the primary of a transformer. The transformer had been split into two and the two halves are connected by a couple of meters of iron wire. Out of the secondary of the transformer appears an electric current. What this shows is that there is no need for electrons to drive our appliances and that the energy we call electricity can be transferred via the magnetic field of a transformer alone. Our homes could be wired with iron wires and our electrical appliances run from a transformer secondary, completely removing the need for electric current in the house wiring. It is but a short step to imagine the utility company supplying energy by magnetic impulse alone; no insulation required! 48
“Let’s add a core! Barium Titanate should work. Or PZT ceramic (Lead Zirconate Titanate.) Our “coil” should attract such a core, which means we could build a solenoid actuator. Or a motor. Or just use the PZT core to pick up certain things. Things like lint, and little bits of paper. It’s not an electromagnet, it’s an electro-electret!” 49
We start to realise that the idea that the electron is a particle or a wave or that it even exists, is a non-question and totally irrelevant as regards any of our existing technology. It makes not a jot of difference to anything that affects the lives of the average person. A scientist will tell us, with the usual circular logic, that all of our electronic technology is thanks to the discovery of the electron by JJ Thomson. It makes sense to those who believe the revisionism, but as we can see from the history in these pages, most of today’s technology was already around in its basic form before anyone had ever heard of JJ.
What has been achieved in the intervening years is development.
Neither Faraday, Crookes, Geissler, Tesla, Braun or Hertz used the word electron before it was made popular by Thomson. But we nevertheless see the fruits of their labours every day. The idea that technological invention or development has to wait for scientific theory is wrong, misleading and dishonest.
The discovery of the Higgs Boson will not affect the lives of the population in any way and is highly unlikely to lead to any improvement in technology. Such things are only important to scientists in as much as they help to keep them employed. The author has no problem with scientists studying irrelevant subjects, but what is a problem is educating and brainwashing us all to think that such things are important in our own lives. The work of science is largely the Emperors New Clothes and all those who can’t see them are supposed by science to be somewhat naive?